Complete step by step answer. Now if we look at benzaldehyde the molecular formula of benzaldehyde is textC_text6textH_text5textCHO.

Draw A Resonance Structure Of The Following Benzaldehyde Chemistry Shaalaa Com
Draw a resonance structure of the following.
. F CH 3 CH CH CH 2. The resonance contributors for the chlorobenzene. We review their content and use your feedback to keep the.
D The structure of C 6 H 5 CHO is. It is basically an aldehyde group substituted with one of the hydrogen atoms of benzene rings. Resonance structure.
Fundamental Concepts in Organic Reaction Mechanism - Resonance Structure. Draw resonsnce structures of the three possible carbocation intermediates to show how an acetyl group CH3CO directs bromination toward the meta position. Its chemical formula is C6H6.
The structure of C 6 H 5 CHO is. Who are the experts. Resonance structure of nitro benzene The presence of an electron withdrawing group which has a double bond adjacent to the phenyl ring of nitrobenzene causes the aromatic ring of nitrobenzene to have a lower electron density than that of benzene as shown by the resonance structures of nitrobenzene.
Is the aldehyde group electron donating or electron withdrawing. There are in total four resonance structures. Explanation on resonance structure of benzoic acid.
This product C6H5-NO2 is windly used for preparation of different types of dyes. E C 6 H 5 CH 2. Answer not in Detail.
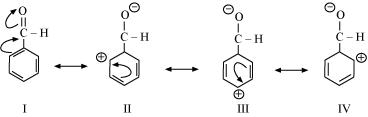
Draw the two possible resonance structures for benzene. Advertisement Remove all ads. The resonance structures of phenol C 6 H 5 O H are as shown.
And this is more useful products for chemical industry. I Aniline is an aromatic amine in which the group is directly attached to. Video Explanation Solve any question of Organic Chemistry - Some Basic Principles and Techniques with-.
Chlorine deactivates the ring and directs ortho and para. Chemistry questions and answers. What is the difference between resonance and chemical equilibrium.
For example benzaldehyde on heating with alc. EC6H5CH2 The resonating structures of the given compound are. The structures contain a negative charge on oxygen and positive charge on the carbon present in the benzene ring.
What is the M and -M effect. It is defined as when more than one Lewis structure can be drawn the molecule or ion is said to have resonance. The resonating structures of benzaldehyde are represented as.
The following image is an explanation of the resonance of benzoic acid. Draw the four resonance structures for anthracene. Draw resonance structures of the following.
The resonating structures of benzaldehyde are represented as. Explanation On Resonance Structure Of Benzoic Acid. The resonating structures of the given compound are.
Resonance structures are a group of two or more Lewis structures that collectively represent a single polyatomic species electronic bonding including fractional bonds and fractional chargesIn organic chemistry benzene is a very common aromatic hydrocarbon. Draw all secondary resonance structures for benzaldehyde. The resonance structures of benzaldehyde c6 h5 cho are as shown.
The replacement of hydrogen atom of benzene ring by alkyl group in presence of anhydrous AlCl 3 is called Friedel Crafts reaction. FCH3 CH CH CH2 The resonating structures of the given compound are. The resonance contributors for the benzaldehyde b Lone pair electrons of chlorine donate electrons by resonance.
45 3 16 2 Choose An Option That Best Describes Your Problem. The number of resonance structures varies depending upon the number of electrons it has. The resonating structures of the given compound are.
Resonance is the concept where electrons bonds are delocalized over three or more atoms which cannot be depicted with one simple Lewis structure. For example seven resonance structures can be drawn for benzaldehyde C HsCHO. Draw all secondary resonance structures for benzaldehyde.
Draw correct structure for benzaldehyde. Resonance structures of benzaldehyde are formed due to the electronegativity of oxygen and the delocalised electrons of benzene ring. How many resonance structures can be drawn for ozone.
However in the case. In this case we can draw three reasonable resonance structures for each carbocation that would result by virtue of attack at each of the three possible positions. The resonating structures of benzaldehyde are represented as.
Write resonance structure of benzene. This problem has been solved. The cyclic structure of benzene molecules is made up of alternating.
Benzaldehyde being an aldehyde reduces tollens reagent. Draw the resonance structure for 1 Benzaldehyde 2 C6H5CH2 3 C6H5CHO - Chemistry - Organic Chemistry Some Basic Principles and Techniques. See the answer See the answer See the answer done loading.
Because three of them place a positive charge on a carbon atom of the benzene ring a CHO group withdraws electron density from a benzene ring by a resonance effect. Experts are tested by Chegg as specialists in their subject area. It is also use for preparation of important chemicals.
Iv Friedel Crafts reaction. Write resonance structure of phenol. Was this answer helpful.
Write resonance structure of benzaldehyde. Aniline and benzaldehyde combines to form a c6h5nchh5c6 type of imine. He resonating structures of the given compound are represented as.
Try drawing out the resonance structure of benzaldehyde to illustrate why what you are saying is not correct. Which position s on the aromatic ring are most likely to react with an electrophile. How many resonance structures can be drawn for N_2O.
What are examples of electron releasing and electron. Draw correct structure for benzaldehyde.

Draw All The Possible Resonance Structures For Benzaldehyde
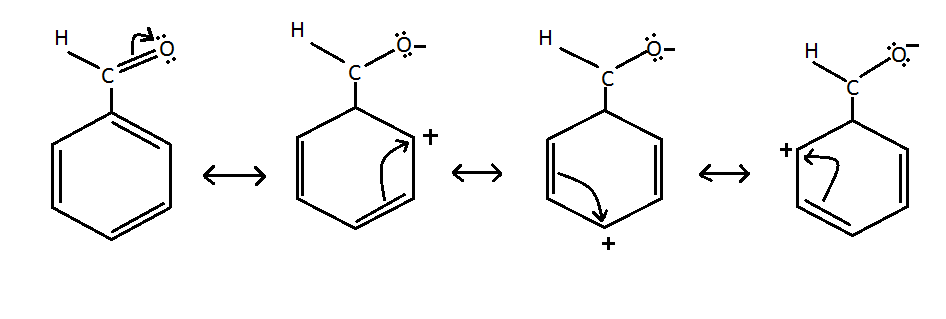
Draw All The Possible Resonance Structures Of Benz Class 12 Chemistry Cbse

Answered Draw The Resonance Structures Of I Phenol Ii Benzaldehyde Iii Aniline Brainly In

Draw The Resonating Structure Of Benzaldehyde Scholr
What Are The Resonance Structures Of Anisole And Benzaldehyde Socratic
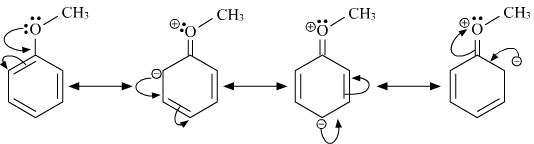
What Are The Resonance Structures Of Anisole And Benzaldehyde Socratic

Super Trick Resonance Structures Of Benzaldehyde Resonance In Benzaldehyde Youtube
0 comments
Post a Comment